CO2– and CH4-CCS by iron salt aerosol (ISA)
CO2– and CH4-CCS by iron salt aerosol (ISA): Sustained carbon burial within the oceanic sediment by coal power plant fluegas, traffic systems and “cloud whitening”
ISA Booklet PDF (german) ISA Booklet PDF (english)
Franz D. Oeste, gM-Ingenieurbüro, D-35274 Kirchhain, Germany; Ernst Ries, Ries Consulting GmbH u. Co. Betriebs KG, D-36154 Hosenfeld, Germany
Poster presented at the 2nd International Conference on Energy Process Engineering (ICEPE 2011) – Efficient Carbon Capture for Coal Power Plants June 20-22, 2011 in Frankfurt /Main, Germany
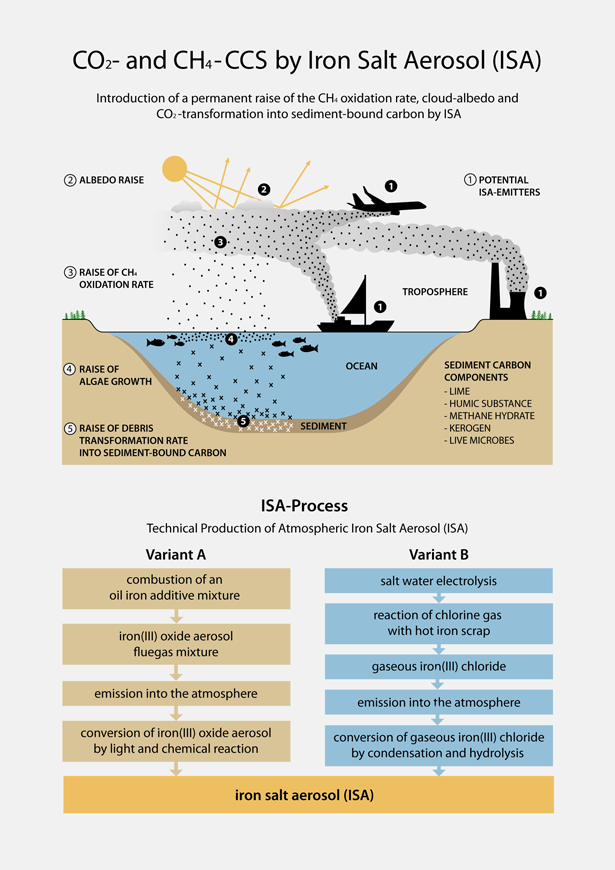
The operation of the ISA process aims to capture and store the CO2 and CH4 carbon (CCS). With the exception of the of iron salt aerosol (ISA) generation the technical ISA-Process is almost identical to the carbon sequestering process that determined the ice age carbon cycle. The ice age carbon cycle was accelerated by the increased transfer- and burial-rate of atmospheric CO2 and CH4 carbon into the oceanic sediments. The lowered greenhouse gas level dropped the climate temperature. The ice age climate was generated by excessive elevation of dust, developing in small parts into ISA. The technical ISA-Process mimics this ice age process by technical generation of ISA.
The ISA triggered the carbon burial and activated the glacial epoch. Data collected from Antarctic and Greenlandic ice core samples, dating back approximately 800,000 years, give transparency to definite correlations between the atmospheric contents of dust, both greenhouse gases CO2 and CH4 and the temperature:
High dust content epochs (up to 50 times of present day levels) pushed the concentration levels of CH4, CO2, as well the overall global temperature, down to glacial levels. Contrary, the warm inter-glacial periods are characterized by low dust or even dust-free periods. These periods are characterized by high CH4– and CO2-levels resulting in temperature increases within Europe and North America to subtropic conditions1A). In reference to the glacial-interglacial CO2 change John C. Martin published his famous “Iron Hypothesis”1B) concerning the biological activation of green plankton growth by iron.
The reasoning behind this phenomenon are small amounts of ISA contained within the dust. As powerful oxidation tool within the troposphere and fertilizing tool at the ocean surfaces ISA accelerated the transfer of the gaseous carbon phases from the planets atmosphere into the condensed carbon state within the oceanic sediments. The natural ISA-driven carbon burial process, may be devided into five definite steps:
1) ISA generation occurs through dust production within the troposphere as a result of aeolic movement of soil particles. Atmospheric ingredients, such as sulphur- and chlorine-compounds, NOx, organic acids and water vapour change small reactive iron oxide fractions of every dust particle into photo-oxidant acting water soluble iron salts (ISA).
2) The ISA content triggers a drop of the atmospheric CH4 concentration level by generation of hydroxyl and chlorine radicals. These are the only oxidants in the lower atmosphere with the ability to initiate methane oxidation. ISA generates the radicals mainly during daytime by the Photo Fenton oxidation cycle and during nighttime by the Fenton reaction. Essentials for the Fenton reaction beside ISA are tropospheric chloride, hydrogen peroxide and sunlight. The Photo Fenton reaction is known as most powerful oxidizing tool. It has received much attention in different technical processes: Decomposition of resistant organics in drinking water, wastewater, tooth whitening, chlorine production, photography2-8).
Additional, tropospheric organics of low vapour pressure adsorb on ISA and become photo-oxidized by ISA. In the absence of ISA these organics consume the radical oxidants hydroxyl and chlorine. This kind of ISA action lowers the atmospheric radical oxidant consumption followed by an increased radical level. This level elevation of the CH4 radical oxidants hydroxyl and chlorine additional triggers the CH4 level drop. Additional the elevated halogene radical level lowers the tropospheric concentration of ozone, a further greenhouse gas.
The main sources of the ISA-generated chlorine radicals are sea spray chloride and hydrogen chloride. Hydrogen chloride, produced by the CH4 chlorine radical reaction, is washed out from the atmosphere by precipitation in the absence of ISA. During ISA epoches hydrogen chloride is re-oxidized to chlorine radicals by the ISA Photo-Fenton-Cycle preventing chlorine loss from the atmosphere by the wash-out of hydrogen chloride9).
3) Dust and ISA content within the dust aerosol initiate a denser and wider spread cloud cover by multiplying the cloud condensation nuclei10). This initiates an additional climate cooling effect by increasing the level of sunshine reflection.
4) After several days and weeks the ISA has left the troposphere by sorption on cloude ice crystals precipitating as rain or snow all over the ocean surfaces. From the water surface the dissolved iron salt distributes within the oceanic photic zone. The ISA precipitation is quasi-continuous throughout extremely wide spread areas, generating very low specific iron input per ocean surface square by ISA. At this point CCS starts. As a consequence of the very high fertilization factor of dissolved iron on the mid-oceanic green plankton, however, due to the very low specific input per square, the following phyto plankton mass growth is small, and at fractions of lower than the 1 % level. Calculating the huge oceanic surfaces, where ISA realizes mass growth, 1 iron atom drives the transfer of up to 100,000 CO2 carbon atoms from the gaseous phase into the condensed organic phase11). Even such low growth increase rates may transfer huge CO2-carbon masses into condensed organic carbon. Phyto plankton represents the first link of the oceanic food chain; consequently the mass increase will cover the whole chain. Additional to the CO2 carbon transfer increase into organic carbon the ISA fertilization generates an increase in CO2 carbon transfer into carbonate carbon bound within skeletons, shells, corals and further carbonate bio-constructs.
5) The increase of sedimentation rate of the organic and carbonate carbon bound to dead organism on the ocean floor, from the former tropospheric CO2 and CH4 carbon will follow at least the same percentage that the iron-triggered oceanic food chain growth has increased. Due to its lower deep water concentration, the availability of free oxygen is restricted near the ocean floor. Due to the ISA-induced increase of the rain of oxygen-consuming organic carbon the oxygen-deficient anaerobic sediment localities at the ocean floor will spread. According to this fact anaerobic micro organisms will change sediment interior and even sediment surfaces of additional localities into anaerobic condition reducing the reoxidation of the organic load. This again will increase the organic sediment load, changing it into kerogene, humic acids and CH4 hydrate. Additional the anaerobic conditions increase the alkalinity to basic conditions by sulphate reduction to hydrogen sulfide and by nitrate reduction to ammonium and nitrogen. These pH changes to basic will trigger the precipitation of additional amounts of CO2 amounts within the anaerobic localities as lime. Reduction of every sulphate or nitrate ion will induce the transfer of at least one additional CO2 molecule into this carbonate precipitate.
The technical ISA-Process
As mentioned above, except the ISA generation step, the world-wide patented ISA-Process runs on a rather identical track. The technical ISA generation may be realized by the variants 1a and 1b:
1a) A flow of a carrier gas like the flue gas of fossil power plants or of traffic vehicles or of any other carrier gas stream is enriched by iron oxide aerosol. This iron oxide aerosol is produced by the well known burning of mixtures of iron-organic oil additives, preferable ferrocene, with oil. The iron-organic oil additives are changed by combustion into iron oxide aerosol. This is composed of nanometer-sized pure iron oxide particles of outstanding chemical reactivity. After emission into the atmosphere, the iron oxide aerosol particles change quantitative and without any residue into water-soluble particulate and/or droplet ISA by reaction with the fluegas- and air-borne chemicals SO2, NOx, water, oxygen, chloride and organic acids like oxalic acid and catechols.
1b) A flow of carrier gas like the flue gas of fossil power plants or traffic vehicles or any other carrier gas stream is enriched by injection of gaseous iron(III) chloride. Within the carrier gas iron(III) chloride change into ISA by condensation and hydrolysation. Preferable the gaseous iron(III) chloride is produced at the emission locality by simple reaction of hot iron scrap with gaseous chlorine. Chlorine can be produced there by salt water electrolysis. This kind of ISA production may utilize the electric energy during day times of low electric power consumption.
All following steps of the technical ISA-Process are in principle the same as in the glacial ISA process described above. Differing to nature the efficiency of the technical ISA process is by orders of magnitude higher than that of natural dust. Some reasons why ISA shall have orders of magnitude higher efficiency than natural dust are:
-
Artificial ISA is composed of nanometer-sized particles; natural dust particle diameters are of micrometer size. This effect raises the chemical activity of artificial ISA.
-
Due to their low velocity free falling artificial ISA particles stay and react much longer than natural dust particles within the troposphere. This effect elongates the duration of chemical activity raising the oxidation capacity per ISA iron atom.
-
Artificial ISA are composed of pure iron salt. The iron salt content of natural dust particles is much lower than 1 percent. This effect raises the chemical activity of artificial ISA.
-
Artificial ISA are easy dissolvable as requirement for consumability by the phyto plankton. Only small parts of the iron mineral fraction within natural dust are soluble. This effect raises the fertilizing capacity of artificial ISA.
We have different clues to calculate rough estimations about the efficiency of the ISA-process11-14):
a) 1 Atom of ISA iron has the ability to sequester up to 100,000 Atoms of CO2-carbon; corresponding to 1 kg ISA iron (=17.86 mol) sequestering up to 79 t CO2 (=1,786,000 mol)
b) 1 Atom ISA iron has the ability to initiate the oxidation of up to 100,000 CH4 molecules; corresponding to 1 kg ISA iron (= 17.86 mol) oxidizing 29 t CH4 (=1,786,000 mol). Within this calculation it should be considered, that the global warming potential (GWP) of CH4 is more than 21 times that of CO215) 16). 29 t of CH4 have a GWP of about 600 t CO2.
From this estimation we can calculate the possible quantitative potential and economy of the order of magnitude of the ISA-Process concerning its ability to oxidise CH4 into CO2 and its ability to bury CO2-carbon. Taking the above calculation we come to the conclusion, that 1 kg of iron in the form of ferrocene may eliminate up to about 600 to 700 t of CO2-equivalents. 1 kg of iron corresponds to about 3 kg ferrocen oil additive or to about 3 kg iron(III) chloride. Then 1 kg of ferrocen or 1 kg of iron(III) chloride shall bury the carbon of up to about 100 to 200 t CO2 equivalents. Calculating the price of ferrocene to about 50 €/kg, and the price of iron(III) chloride to lower 5 €/kg, the costs to bury the carbon of 100 t CO2 equivalents without any harm to the environment and to the health of men will be even lower than 100 € in the case of ferrocene as ISA educt and even lower than 10 € in the case of iron(III) chloride as ISA educt.
In the order to bury the whole of CO2 emission quantity of a coal power plant by ISA the necessary concentration of the ISA precursor alternatives iron(III) oxide or iron(III) chloride within the flue gas may fall short of 1 mg/Nm³ Fe; this would even apply in a brown coal power plant flue gas. Because the carbon burial location is independent on the greenhouse gas emission location, the ISA Process may be carried out in remote areas on the ocean or within deserts to keep away any harm to human health.
Climate chamber and bench scale research will have to be done to realize stable and quantitative data as a base for certification of the ISA process as CCS process. Certified, the ISA process would be a very useful tool for potential process owners and licence holders by compensating or even selling eco credits.
The ISA process has even the potential to overcome the climate warming problem and may be defined as geo-engineering process. Combined with the off-shore “cloud whitening” geo-engineering process17) by unmanned sea salt spray ships, the ISA process could improve the physical cloud whitening cooling by addition of the chemical and biological carbon burial of greenhouse gas carbon within an sustained ocean sediment storage as fuel- and lime-carbon sediment. The sea salt aerosol carrier gas stream of the cloud whitening process additional may act as carrier gas for ISA as well. This combined ISA cloud whitening action shall result in an extraordinary improvement of both processes: By the enrichment of ISA with sea salt chlorine the ISA process would gain effectivity. The cloud whitening process to reduce the global temperature would gain drastic improvement of economy and even ecology; we estimate that this advantage could reduce the number of the proposed fleet of sea salt spray ships for cloud whitening from the proposed 1,500 ships to 10 or lower, gaining as well CCS extension plus extension of the albedo increase by cloud cover whitening and increase.
References
- A) van der Pluijm, B.A & Sefcik, L.T. 2007: Inquiries in global change, Unit 8a. Analysis of Vostok ice core data; November 14, 2007: http://www.globalchange.umich.edu/globalchange1/current/labsLab9/Vostok.htm
B) Martin, J.H. 1990: Glacial-interglacial CO2 change: The iron hypothesis; Paleooceanography. 5(1), pp. 1-13 - Sattler, C. 2003: SOWARLA Solare Wasserreinigung von der Entwicklung in die Praxis; Deutsches Zentrum für Luft- und Raumfahrt (DLR) Inst. F. Technische Thermodynamik Solarforschung, Köln-Porz; www.ttz-lampoldshausen.de/ximages/27763praesentat.pdf
- Prousek, J. et al. 2007: Fenton and Fenton-like AOPs for wastewater treatment: From-laboratory-to-plant-scale-application; Separation Science and Technology; 42(7), pp. 1505-1520
- Anonym – Chairside Zahnaufhellungssystem 2009: „Die beste Zahnaufhellung die wir je hatten“; Druckschrift der Diskus Dental Europe B.V., 76275 Ettlingen; www.discusdental.com/de
- Ling M. et al. 2006: Photochemical synthesis of chlorine gas from iron(III) and chloride solution; Journal of Photochemistry and Photobiology A: Chemistry, 183(1-2), pp. 126-132
- Eder, J.M. 1880: Über die Zersetzung des Eisenchlorids und einiger organischer Ferridsalze; Monatshefte für Chemie, 1, Dezember 1880, pp. 755-762
- Eder, J.M. 1881: Decomposition of ferric chloride and some ferric salts of organic acids by light; J. Chem. Soc. Abstr. 40, pp. 670-671
- von Hübl, A.F. 1895: Der Platindruck, 1. Abschnitt. Das Platinpapier und seine Herstellung; Druck und Verlag von Wilhelm Knapp
- Oeste, F.D. 2004: Climate cooling by interaction of natural or artificial loess dust with tropospheric methane; GeoLeipzig 2004, Joint Conference on the German Geological Society (DGG) and the Society of Geo-Sciences (GGW), 29th Sept. – 1st Oct. 2004, Leipzig.
- Rosenfeld, D. et al. 2008: Flood or drought: How do aerosols affect precipitation? Science, 321, pp. 1309-1313
- Duggen, S. et al. 2007: Subduction volcanic ash can fertilize the surface ocean and stimulate plankton growth: Evidence from biogeochemical experiments and satellite data; Geophysical Research Letters, 34 L01612, doi:10.1029/2006GL027522, pp. 1-5
- Fabian, P. 1984: Atmosphäre und Umwelt; pp. 72-73, Springer-Verlag; ISBN 3-540-55773-3 – Anmerkung: P. Fabian counts a methane growth rate of about 2 % per year. After eruption of the iron salt bearing ash from mount Pinatubo in 1991 the methane growth rate dropped to zero. Then again the growth rate changed to positive values.
- Australian Greenhouse Office 2005: Australian Government Department of Environment and Heritage Australian Greenhouse Office: Have methane levels stabilized? Hot topics in climate change science, topic 10, fig. 1, www.greenhouse.gov.au/science/hottopics/pubs/topic10.pdf
- Gauci, V. et al. 2008: Halving of the northern wetland CH4 source by a large Icelandic volcanic eruption; J. Geophys. Res., 113, G00A11,doi:10.1029/2007JG000499
- Fabian, P. 2002: Atmosphäre und Umwelt; pp. 24, Springer-Verlag; ISBN 3-540-43361-6
- Völker-Lehmkuhl, K. 2005: Praxis der Bilanzierung und Besteuerung von Emissionsrechten, p. 9, Erich Schmidt Verlag; ISBN 978-3-505-09310-6
- A) Latham, J. 2002: Amelioration of global warming by controlled enhancement of the albedo and longevity of low-level maritime clouds; Atmos. Sci. Lett. 3, pp. 52-58
B) Salter, S., Sortino,G. Latham, J. 2008: Sea-going hardware for the cloud albedo method of revising global warming; Phil. Trans. R. Soc. A366(1882), pp. 3989-4006